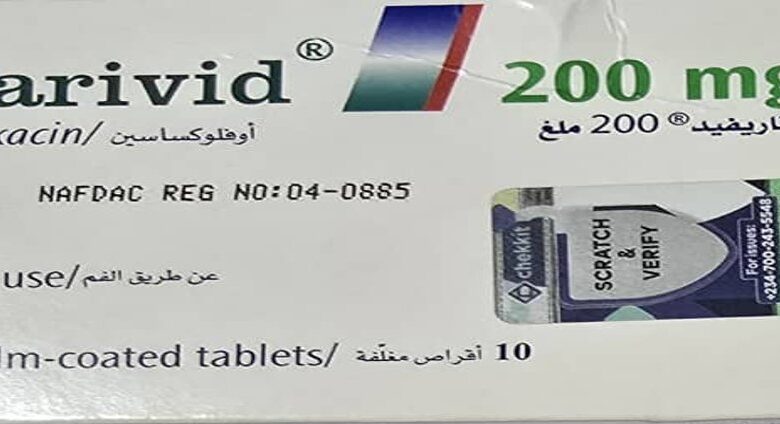
The National Agency for Food and Drug Administration and Control (NAFDAC) has alerted Nigerians about the presence of an unregistered batch of Tarivid 200mg in the country.
NAFDAC disclosed this on Monday, noting that the product was discovered during a routine surveillance conducted in the Onipan area of Shomolu Local Government Area, Lagos State.
“Upon investigation, it was confirmed by a representative of the product’s Marketing Authorisation Holder (SANOFI) that the unregistered batch identified during the surveillance was not intended for the Nigerian market, but rather for the Pakistani market.
“This conclusion is a result of a review conducted on the batch by Sanofi’s Anti-Falsified and Illicit Trafficking (AFIT) Central Lab. It was also stated that the batch is not authorised for distribution in Nigeria and does not fall under the scope of a validly registered product with NAFDAC,” the regulatory body siad.

It explained that Tarivid is a brand of Ofloxacin, which is an antibacterial agent used for the treatment of bacterial infections in many parts of the body, including the respiratory tract, kidney, skin, soft tissue, and urinary tract.
The agency advised healthcare professionals and consumers to report any suspicion of the sale of substandard and falsified medicines or medical devices to its nearest office.
It also urged them to call 08001623322 or send an email to sf.alert@nafdac.gov.ng.








916ff09
Search engine penalty recovery procedures and top practices